The Axolotl is an excellent model to explore regeneration. However, the examination of its nervous system has so far been difficult because the suitable tools for visualization and manipulation of neuronal circuits were missing. In a study published in PNAS, Katharina Lust and Elly Tanaka from the Institute for Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences are now presenting a method with which genes can be specifically introduced into Axolotl neurons. This makes it possible to make the neural organization of the Axolotl visible and to examine it in detail.
The exceptional regenerative skills of the Axolotl (Ambystoma mexicanum) – This type of salamander can grow back and repair complex organs such as the retina and the brain – make the Axolotl an ideal model in order to examine both the formation of neuronal circuits and its regeneration after an injury. So far, the brain regeneration in Axolotl has been researched with classic methods, for example by using tracers and antibodies. However, the researchers have been lacking: so far the tools to grasp the dynamics of regeneration of neuronal circuits, to examine the functional regeneration and to specifically manipulate the neuronal function in the Axolotl brain.
Now Katharina Lust and Elly Tanaka from the Institute for Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences present an efficient method in which genes are introduced into axolotl neurons using certain viruses. This enables researchers to be dynamically visualized and deliberately transferred the nerve cells into nerve cells. Her results were published in PNAs on March 5.
Make Axolotl neurons shine
Gene can be introduced into cells using various methods, for example by using harmless viruses as gene ferries. So far, however, it had not been possible to inert genes through viruses in Axolotl neurons. In the study now published, lust and Tanaka showed for the first time that adeno-associated viral vectors (AAV) can enforce transgenic in Axolotl neurons efficiently. By testing different AAV serotypes – variants that aim at different cell types – the scientists identified the most suitable serotype for the transmission of transgenes into Axolotl neurons.
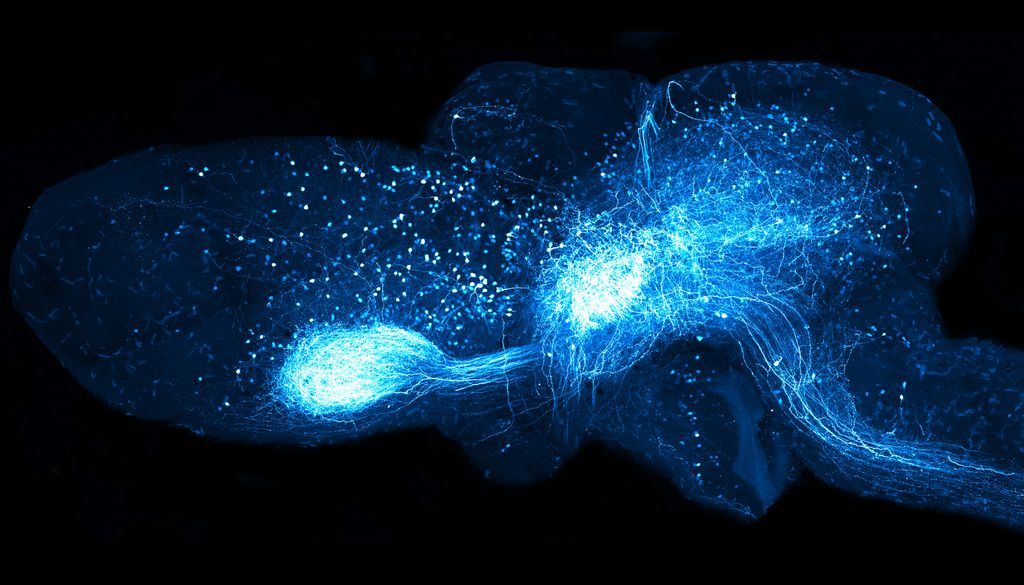
With the help of this method, the scientists smoke the fluorescent marker GFP in nerve cells of a living axolotl. As a result, the researchers were able to mark various types of neuron.
From the eye to the brain – and back
The visualization of neuronal connections enables scientists: inside, map the circuits that link different brain areas. With the help of the viral introduction of GFP into the axolotl retina, lust and tanaka were able to map the connections and forward visual information to different brain regions via retina neurons. In addition, they also identified neural projections in the opposite direction – from the brain to the retina -, which indicates that the brain influences the function of the retina and is fine -tuning.
“This technology opens up a new way to make neuronal activity in Vivo visible in the brain and to follow how neuronal circuits regenerate after an injury,” explains first author Katharina Lust, postdoctoral student in the laboratory of Elly Tanaka.
Breakthrough for Axolotl brain research
In addition to the possibility of dynamically visualizing neurons in the Axolotl brain, this study establishes viral vectors as powerful tools in order to bring in specific genes in Axolotl neurons and to examine the neuronal organization.
“Viral vectors could be used to manipulate neural circuits or to research the role of specific genes in regeneration of the Axolotl brain,” explains Elly Tanaka, scientific director of the IMBA and corresponding author of the study. “This tool opens experimental possibilities that were previously unreachable at the Axolotl. The Axolotl is thus establishing itself as a central vertebrate model in the molecular neuroscientific and helps us to better understand the essential properties of the vertebrate brain. ”